NA Labetalol Hydrochloride Impurity - Anax Laboratories
Por um escritor misterioso
Descrição
Anax Laboratories provides Chemical industry users with Impurities of Labetalol Hydrochloride Impurity(NA) Boiling point Melting point, Labetalol Hydrochloride Impurity (NA ) Density MSDS Formula Use,If You also need to Labetalol Hydrochloride Impurity (NA ) Other information,welcome to contact us.

TODO ORO BAJA CALIFORNIA 5
NA Enalapril Impurity - Anax Laboratories

USP 'S Workshop On Nitrosamines Impurities: Analysis, Industry Needs and Regulatory Perspectives, PDF, Ion
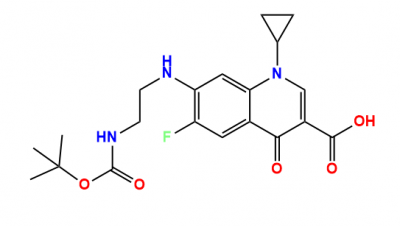
Anax Laboratories
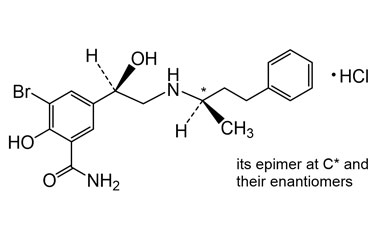
CAS No : 2445226-37-7, Product Name : Labetalol Hydrochloride - Impurity G (Hydrochloride Salt)
Pfizer Hospital US

O&M Halyard Inc 55093 - McKesson Medical-Surgical

Nembutal Sodium Solution Barbiturate
(Anxiolytic / Sedative / Hypnotic)
Pentobarbital Sodium, Preservative Free
50 mg / mL Intramuscular or Intravenous Injection
Multiple Dose Vial 20 mL CII
Akron 76478050120
(Anxiolytic / Sedative / Hypnotic)
Pentobarbital Sodium, Preservative Free
50 mg / mL Intramuscular or Intravenous Injection
Multiple Dose Vial 20 mL CII
Akron 76478050120

logo.jpg
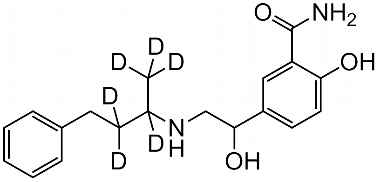
Product Name : Labetalol Hydrochloride - Impurity E, Chemical Name : Mixture of 3 Stereoisomers of 5,5′-piperazine-2,5-diylbis(2-hydroxybenzamide)
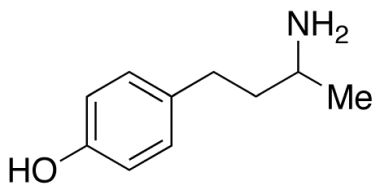
CAS NO : 2726492-68-6, Product Name : Labetalol Hydrochloride - Impurity A (Hydrochloride Salt), Chemical Name : Mixture of 4 stereoisomers of 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl)amino]ethyl]benzoic Acid Hydrochloride

Amneal Pharmaceuticals LLC Issues Voluntary Nationwide Recall of Metformin Hydrochloride Extended Release Tablets, USP, 500 mg and 750 mg, Due to Detection of N-Nitrosodimethylamine (NDMA) Impurity

USP 'S Workshop On Nitrosamines Impurities: Analysis, Industry Needs and Regulatory Perspectives, PDF, Ion
de
por adulto (o preço varia de acordo com o tamanho do grupo)