Pathogens, Free Full-Text
Por um escritor misterioso
Descrição
In Brazil, blood donation is regulated by the Brazilian Ministry of Health, and all States follow the same protocol for clinical and laboratory screening. Brazil is an endemic country for Chagas disease (CD), caused by Trypanosoma cruzi, and for leishmaniasis, caused by a species of Leishmania spp. Screening for leishmaniosis is not routinely performed by blood banks. Given the antigenic similarity between T. cruzi and Leishmania spp., cross-reactions in serological tests can occur, and inconclusive results for CD have been found. The objective of this study was to apply molecular techniques, e.g., nPCR, PCR, and qPCR, to clarify cases of blood donation candidates with non-negative serology for CD and to analyze the difference between the melting temperature during real-time PCR using SYBR Green. Thirty-seven cases that showed non-negative results for CD using chemiluminescent microparticle immunoassay (CMIA) tests from blood banks in Campo Grande, MS, and Campinas, SP, were analyzed. In the serum samples, 35 samples were evaluated by ELISA, and 24.3% (9/35) showed positive results for CD. nPCR was able to detect 12 positive results in 35 samples (34.28%). qPCR for T. cruzi was quantifiable in the samples that showed a value ≥0.002 par eq/mL (parasite equivalents per milliliter), and in 35 samples, 11 (31.42%) were positive. Of all evaluated samples using the described tests (CMIA, ELISA, nPCR, and qPCR), 18 (48.6%) were positive for CD. For MCA by qPCR, the melting temperature was 82.06 °C ± 0.46 for T. cruzi and 81.9 °C ± 0.24 for Leishmania infantum. The Mann–Whitney test showed a significant value of p < 0.0001. However, the differentiation between T. cruzi and L. infantum could not be considered due to temperature overlap. For leishmaniasis, of the 35 samples with non-negative serology for CD tested by the indirect fluorescent antibody test (IFAT), only one sample (2.85%) was positive (1:80). The PCR for Leishmania spp. was performed on 36 blood samples from donation candidates, and all were negative. qPCR for L. infantum showed 37 negative results for the 37 analyzed samples. The data presented here show the importance of performing two different tests in CD screening at blood banks. Molecular tests should be used for confirmation, thereby improving the blood donation system.

Solved 1. What is epithelia, what is an example of
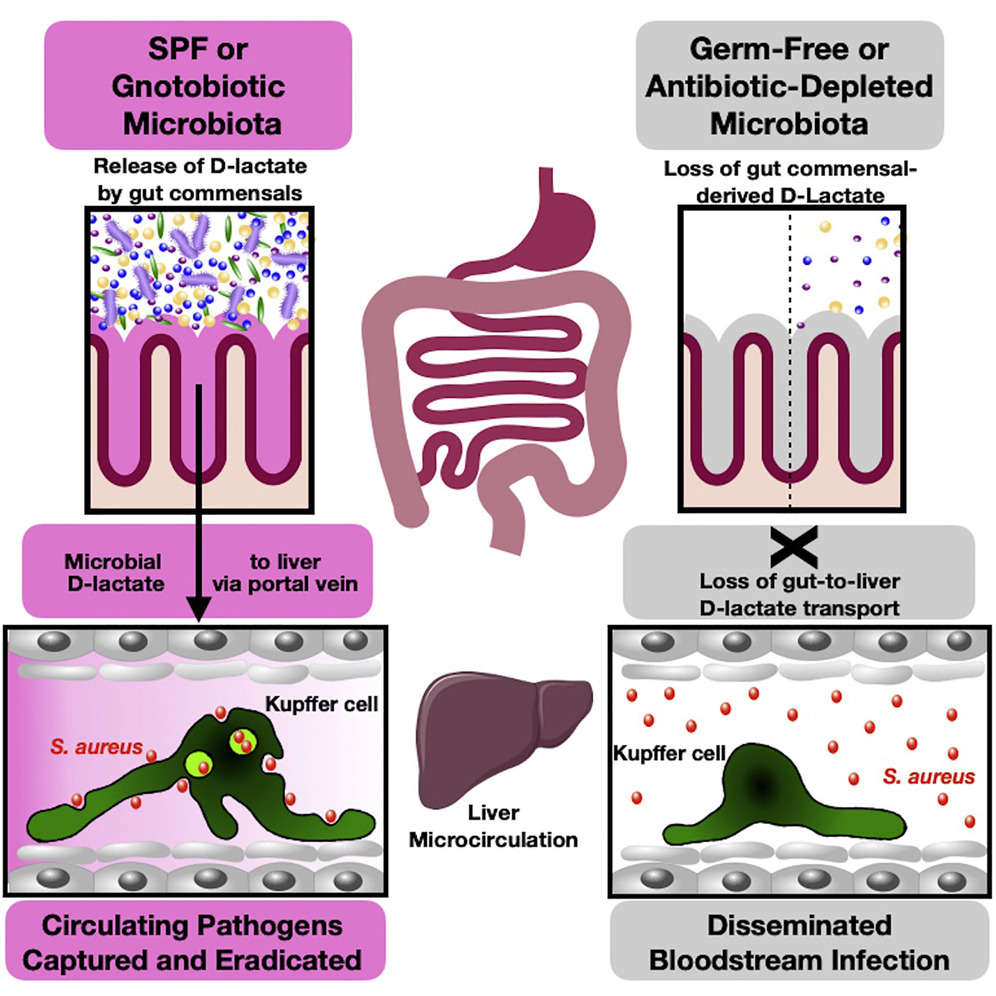
Cell Host & Microbe on X: Microbiota-Driven Firewall Stops Pathogens: @BraedonMcdonald & Co show that pathogen clearance by Kupffer cells is governed by #gut #microbiota & D-lactate, which reaches liver via portal

Hospital-Acquired Infections Due to Gram-Negative Bacteria

NADases as weapons for both plant pathogens and their hosts
Ssa Crystal Reports Flree Trial Download - Colaboratory

Southeastern Naturalist, Volume 15, Number 1 (2016): N4–N6

Read et al. warned us 2015 using chickens (Marek's disease) that using an imperfect 'leaky' vaccine can enhance transmission of highly virulent pathogens; sub-optimal non-sterilizing non-neutralizing
Lg Download Software V1.3.6.3 - Colaboratory

Ecological and socioeconomic factors associated with the human burden of environmentally mediated pathogens: a global analysis - The Lancet Planetary Health
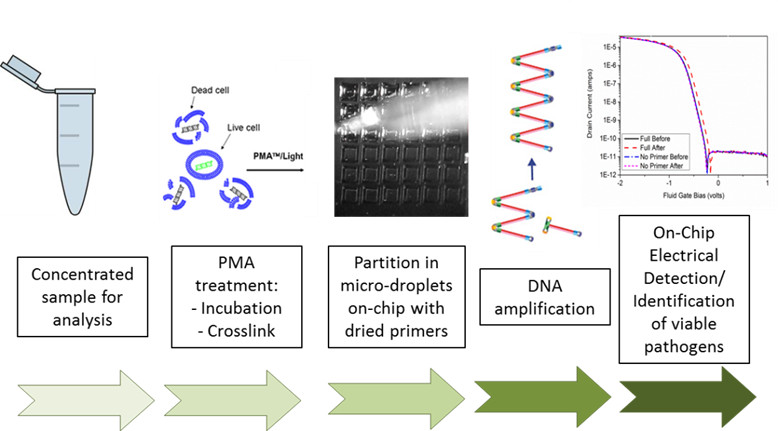
Detection of Viable Pathogens Using Label-Free Electrical Detection of Nucleic Acid Amplification : LIBNA
de
por adulto (o preço varia de acordo com o tamanho do grupo)